1-2 Punch: PD-L1 x 4-1BB et al.: Dual MOA or simply conditional bsAb ?
Takeaway: Immune checkpoint/agonist-targeting bsAb ≠ dual MOA bsAb
-
Takeaway: Immune checkpoint/agonist-targeting bsAb ≠ dual MOA bsAb -
Clinical data for dual checkpoint/immune agonist-targeting bsAbs have so far been satisfactory, but not especially exciting. The most popular combination continues to be PD-L1 x 4-1BB, while combinations of PD1/PD-L1 immune checkpoint targeting with other TNFRSF members (ie CD40 and OX40) agonism is also being explored. The main question for the bsAbs of the kind is whether at administered doses 4-1BB-mediated agonism can be significantly complemented by immune checkpoint blocking (ICB) function or whether PD1/PD-L1 serves solely for conditional activation of TNFR.
Rationale for Combination Approaches: Endogenous vs Synthetic Immunity
Endogenous immune response largely relies on existing previously primed CD8+ T cells that specifically recognize cancer cells. These immune cells can either be re-invigorated or expanded by PD-L1/PD-1 inhibition, leading to recognition and killing of cancer cells, as well as further proliferation of the T cells.
Unlike adaptive immunity, synthetic immunity promotes proliferation of both tumor-resident and non-specific T cells and recruitment to the tumor. Synthetic immune responses are the result of therapeutics that artificially bind T cells to cancer cells that might not normally do so based on their cognate binding of a T cell receptor to a specific peptide-MHC complex. Examples include engineered CAR T cells, CD3 bsAb and immune agonists approaches. Synthetic immune approaches may enable the generation of an initial anti-cancer immune response to cancers that are poorly immunogenic, leading to stimulatory cytokine upregulation, immunogenic cancer cell death, and further activation of endogenous anti-cancer immune responses. However, synthetic immune responses generally lead to IFNγ secretion and increased expression of PD-L1, which can at least partially dampen the synthetic immune response.
Given the above, therapies that combine endogenous and synthetic immune approaches may be particularly synergistic. The majority of cancer either do not expresses a strong enough endogenous immune response (immune excluded and immune desert phenotypes) to eradicate cancer or are resistant (or develop resistance) to synthetic immune approaches, preventing curative outcome. ICB can relieve any immune suppressive effect and further enhance the synthetic immune response (discussed by Hegde and Chen, 2020).
The inflamed, immune-excluded, and immune desert phenotypes are prevalent at varying degrees within a given tumor type and across cancers. Figure illustrates and broadly classifies the approximate phenotype prevalence within each cancer and places them across the tumor immunity continuum as they correlate to TMB.
Immune Agonists
No immune agonists have been brought to market as of yet. While several different mechanisms have reached Ph II or III (4-1BB, OX40, GITR, ICOS, CD27), most with clinical data have had modest efficacy. Dosing has been approached carefully, especially with combination regimens; this has likely been driven by the experience of TeGenero's CD28 superagonist TGN1412, which induced severe inflammatory reactions in 2006. Thus far toxicity has been mild for most targets.
Tumor-targeting 4-1BB bsAbs: Novel Immune Agonists
Newer 4-1BB agonists are focusing activity at the tumor by simultaneously targeting a tumor antigen: 4-1BB TAA bsAbs - 5T4, CD19, BCMA, EphA2, GPC3, HER2, FAP, Nectin4, CLDN18.2, ROR1, MSLN, PSMA/HSA; this may allow for increased activity without increased toxicity.
-
Newer 4-1BB agonists are focusing activity at the tumor by simultaneously targeting a tumor antigen: 4-1BB TAA bsAbs - 5T4, CD19, BCMA, EphA2, GPC3, HER2, FAP, Nectin4, CLDN18.2, ROR1, MSLN, PSMA/HSA; this may allow for increased activity without increased toxicity. -
The majority of new immune agonists target 4-1BB (ie CD137). 4-1BB is an inducible costimulatory receptor expressed on activated T cells that have recently engaged in antigen recognition, supporting their proliferation and enhanced functionality, and natural killer (NK) cells that had been engaged in Ab dependent cellular cytotoxicity (ADCC), so they can be further co-stimulated to more efficiently perform ADCC.
Being a category II TNFR, 4-1BB requires clustering to induce signaling. First generation of 4-1BB Abs cannot achieve clustering, unless they are themselves hyper-crosslinked via FcγR on adjacent cells.
While first generation 4-1BB activating Abs demonstrated potent anti-tumor responses in preclinical models, 4-1BB agonizing Abs have thus far failed to progress beyond early clinical either due to hepatic toxicities caused by FcγR-crosslinking (urelumab, IgG4; Segal et al., 2017) or due to low clinical activity (utomilumab, IgG2).
An initial optimization of 4-1BB Abs for cancer immunotherapy was therefore by balancing agonistic strength with FcγR affinity (Qi et al., 2019). An attractive next generation approach to 4-1BB agonistic approaches present multi-functional biologics which conditionally activate 4-1BB only in the tumor microenvironment and thereby localize activity.
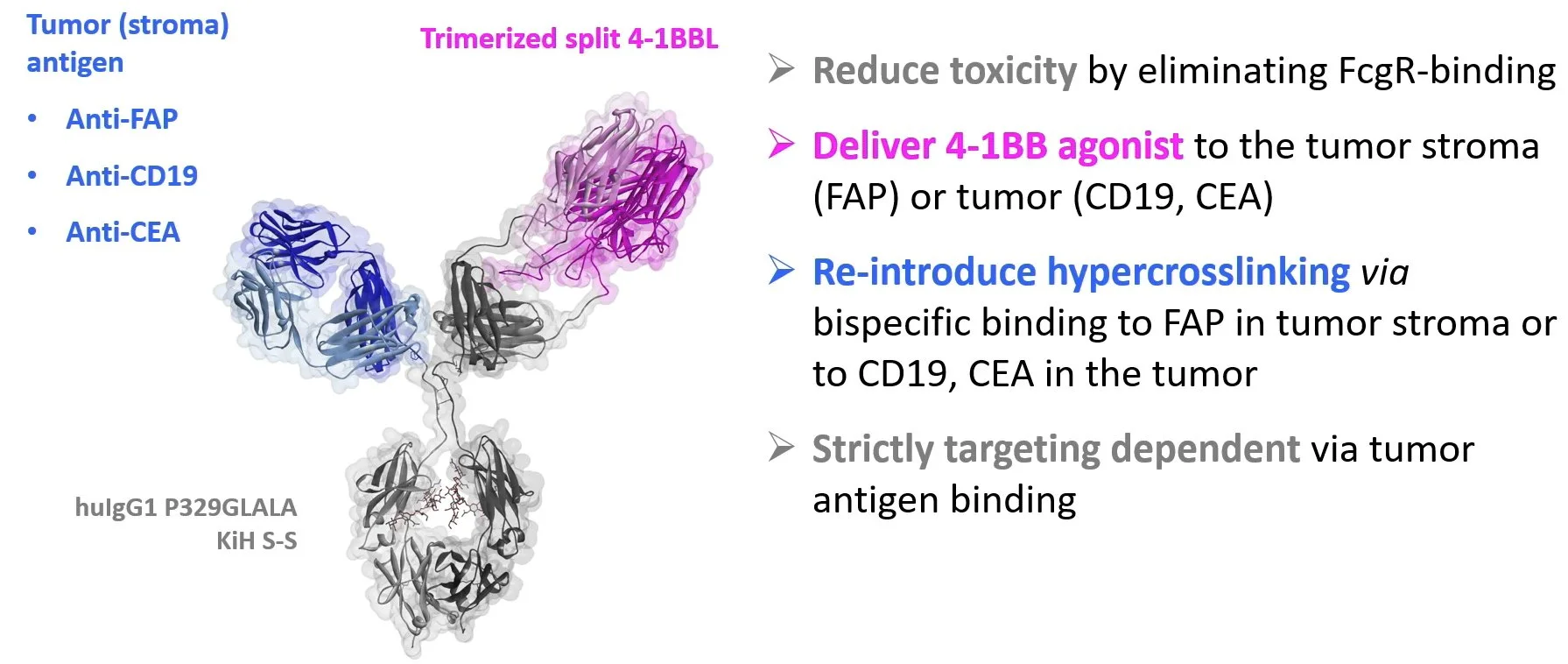
One of the first bispecific constructs developed by Compte et al. 2018 was targeting 4-1BB on T cells without harmful Fc receptor cross-linking, and the tumor-associated antigen (TAA) EGFR, an approach having the limitation of efficacy being expected only on tumors that express such TAA. Similarly, Claus et al. 2019 have taken a protein engineering approach to make next-generation 4-1BB agonist with a trimeric 4-1BBL portion and included more universal tumor antigen–targeting portion against fibroblast activation protein (FAP): FAP-4-1BBL (RG7826, RO7122290). FAP is a common moiety expressed on cancer associated fibroblasts and is present in most tumors.
At AACR2022, Christian Klein from Roche Glycard presented the 4-1BB agonism concept Roche has developed.
4-1BB activation has been shown to upregulate PD-1 expression on effector T cells and PD-L1 on tumors via release of IFNγ, which is linked to therapeutic resistance. The combination of PD-1/PD-L1 pathway blocking antibodies with 4-1BB agonist antibodies in these models synergistically enhanced anti-tumor responses. Given the preclinical data, along with the high frequency of PD-1 and CD137 co-expression on tumor-specific CD8+ tumor-infiltrating lymphocytes (TILs) found in humans, there is a clear mechanistic rationale for dual targeting of the PD-1/PD-L1 axis and CD137 to optimally engage anti-tumor immunity.
The solutions pursued (and under clinical development) to secure maximal efficacy of 4-1BB monotherapy while avoiding liver toxicity show synergy with PD-1 blockade.
FAP x 4-1BB-L (RG7826, RO7122290) has entered the clinic as a single agent and in combination with anti–hPD-L1 mAb atezolizumab in a first-in-human (FIH) trial. Monotherapy activity of RG7826 was limited, with 2 PR observed (Melero I, ESMO2020). Combination with Atezolizumab demonstrated 8 PR.
First-in-human (FIH) phase I study of RO7122290 (RO), a FAP-targeted 4-1BB agonist, administered as single agent : Objective response: 2 PR occurred in part A including thymoma and RCC. (Melero I, ESMO2020)
First-in-human (FIH) phase I study of RO7122290 (RO), a FAP-targeted 4-1BB agonist, , administered in combination with atezolizumab: Objective response: 8 PR occurred in part B/IMG sub-study+ including 2 mesothelioma, 2 SCLC, 2 TNBC, 1 thymoma and 1 Merkel cell carcinoma. (Melero I, ESMO2020)
The study data presented at ESMO2020 included results from the dose-escalation phases of the trial, parts A and B. In part A, where single-agent RO7122290 was administered in 62 patients at doses ranging from 5 mg to 2000 mg, grade 3/4 adverse effects (AEs) occurred in 50% of patients, with the most common being asthenia (6.5%) and AST increase (4.8%). Similarly, in part B 57% of patients experienced grade 3/4 AEs, with the most common being pneumonia (11.3%), pneumonitis (7.5%), ALT increase (5.7%), lymphopenia (5.7%), and neutropenia (5.7%). In total, 3 dose-limiting toxicities (DLT) were observed in parts A and B. irAEs of any grade were reported in 25.8% of patients treated in part A and 37.7% of those in part B. Rash was the most common all-grade irAE in both groups. Corticosteroids were required to treat irAEs in 9.7% and 17.0% of patients in parts A and B, respectively.
Pieris Pharmaceuticals Announced in Jan 2022 Dosing of First Patient in Ph II Gastric Cancer Trial of 4–1BB/HER2 bsAb Cinrebafusp Alfa
-
Pieris Pharmaceuticals Announced in Jan 2022 Dosing of First Patient in Ph II Gastric Cancer Trial of 4–1BB/HER2 bsAb Cinrebafusp Alfa -
Bicycle initiated a Ph I trial of BT7480 (Bicycle TICA targeting Nectin-4 and agonizing CD137) in 4Q2021, and dose escalation in that trial remains ongoing.
-
Bicycle initiated a Ph I trial of BT7480 (Bicycle TICA targeting Nectin-4 and agonizing CD137) in 4Q2021, and dose escalation in that trial remains ongoing. -
Attempt for 2 in 1 Combination Approach: PD-L1 - Targeting 4-1BB bsAbs
An alternative approach to conventional combinatorial immunotherapies is to develop next-generation bispecific immune checkpoint/agonist-targeting immunotherapies. The bsAb with dual targeting of PD-L1 and 4-1BB, would have the potential to activate T cells through PD-L1 blockade and simultaneous conditional 4-1BB co-stimulation. This PD-L1 × 4-1BB bispecific approach has been proposed to offer the advantage of acting on two complementary immune-oncology targets within one compound.
The main question for such proposed dual MOA bsAbs however is whether such bsAbs indeed perform as dual MOA Abs, or in fact act solely as conditional agonists.
Protein engineering strategies to target CD137 (4-1BB) costimulation to the tumor microenvironment include targeting CD137 agonists to TAA, to proteins selectively expressed in the stroma such as fibroblast activated protein (FAP), or using antibodies targeting CD137 that become unmasked and active in the tissue microenvironment (probodies). In other instances, dual targeting of costimulatory and coinhibitory receptors enriched in the tumor microenvironment by a single multi-specific protein is being attempted with potential for synergistic effects in a single moiety
PD-L1 x 4-1BB is the most popular combination of ICB/immune agonist bsAbs in clinical trials (see side bar).
Muik et al. Dec 2021 (BioNTech-Genmab) demonstrated PD-L1 blocking and conditional 4-1BB agonist activity of the mbsAb-PD-L1×4-1BB in multiple in vitro systems and tumor models and anti-tumor activity of mbsAb-PD-L1×4-1BB in vivo, however failed to provide relevant comparison to single MOA 4-1BB activation. Namely, the monovalent control antibody mbsAb-Ctrl×4-1BB cannot cluster 4-1BB receptor efficiently, therefore its direct comparison with mbsAb-PD-L1×4-1BB is not relevant.
Anti-tumor effect of mbsAb-PD-L1×4-1BB is dependent on conditional agonist activity, while the contribution of blocking MOA remains unaddressed. (A) mbsAb-PD-L1x4-1BB is a Fc-silenced bsAb of mAb-PD-L1 and mAb-4-1BB, containing L234A and L235A Fc-silencing mutations that abrogate binding to FcγR and C1q. (B) mbsAb-Ctrl-4-1BB fails to induce 4-1BB signaling, while mbsAb-PD-L1×4-1BB elicits conditional PD-L1-driven 4-1BB activation in a mouse 4-1BB reporter assay. (C) PD-L1×4-1BB was tested in CT26 syngeneic model compared to the monovalent control antibodies mbsAb-PD-L1×Ctrl and mbsAb-Ctrl×4-1BB alone or in combination, or isotype control antibody (mAb-Ctrl). Caveat of the study is that 4-1BB requires clustering to induce signaling (Muik et al. Dec 2021 (BioNTech-Genmab).
At AACR2022, Maria Jure-Kunkel (Genmab) presented GEN1046 (first-in-class PD-L1 x 4-1BB bsAb) as a conditional 4-1BB agonist being investigated for the treatment of advanced solid tumors in two clinical trials (NCT03917381 and NCT04937153) and described its MOA as simultaneous and complementary blockage of PD-L1 and conditional 4-1BB agonism. (Of note, in Nov. 2021 Phase II trial was announced for 2L (post-ICI) with GEN1046 ± pembrolizumab for PD-L1+ mNSCLC; NCT05117242)
GEN1046 demonstrated impressive preclinical functional activity with 9/9 CR in huPD-L1 x 4-1BB knock-in mice bearing MC38/hu-PD-L1 tumors. Further, GEN1046 showed clinical activity in heavily pretreated patients, including cases resistant to prior PD-(L)1 immunotherapy.
GEN1046 clinical activity in heavily pretreated patients, including cases resistant to prior PD-(L)1 immunotherapy: disease control occurred in 40/61 (65.6%) subjects in dose escalation, with 4 PR, including TNBC (n=1), ovarian cancer (n=1) and NSCLC (n=2) patients. (Jure-Kunkel, AACR2022)
Baseline tumor PD-L1 status was associated with tumor reduction with GEN1046 treatment. However these results cannot serve to address whether ICB function is critically contributing to GEN1046 activity, as PD-L1 mediated clustering is required for providing conditional 4-1BB activation.
Clinical activity of GEN1046 by tumor PD-L1 status across CPI-experienced patients with advanced solid tumors (biomarker evaluable only). Greater proportion of patients harboring PD-L1+ tumors exhibited reduction in tumor volume with GEN1046 treatment (n=14/30, 47%) compared to patients with PD-L1neg tumors (n=3/23, 13%).
While GEN1046 was generally well tolerated, TRAE resembled those of TAA x 4-1BB. Dose-limiting toxicities (DLT) occurred in six (9.8%) patients (25 mg, n = 1; 80 mg, n = 1; 140 mg, n = 2; 200 mg, n = 1; 800 mg, n = 1) and included grade 4 febrile neutropenia (n = 2), grade 3 immune-mediated nephritis (n = 1), grade 3 ALT increase (n = 1), grade 3 AST/ALT increase (n = 1), and grade 3 transaminase elevation (n = 1). GEN1046-related Grade 3 transaminase elevations were resolved with corticosteroids. (Muik et al. BioNTech/Genmab, 2022)
GEN1046 treatment-related adverse events (TRAEs) in GCT1046-01 escalation phase. TRAEs of ay grade occured in 70.5% patients, with 27.8 patients experiencing Grade 3-4 TRAEs.
Based on on PK/PD model predictions (Bajel et al. SITC2021), overall evidence from clinical safety and preliminary efficacy, and consistent modulation of PD markers, 100 mg Q3W was chosen as the GEN1046 expansion dose. With optimizing the formation of the trimer and achieving about 70% receptor occupancy in the tumor, the selected dose of GEN1046 for expansion cohorts is lower compared to dose levels commonly used for ICB, which questions whether the level of PD-L1 blocking is acceptable for GEN1046 to be designated as dual MOA bsAb.
GEN1046 dose selection for expansion cohorts based on PK/PD model predicting trimer formation and PD-L1 receptor occupancy across different dose levels. Schematic of in vitro (A) and in vivo (B) semi-mechanistic PK/PD model for GEN1046, and PK/PD model output (C). Maximum levels of trimer complexes predicted at 100 mg Q3W.
To this regards, BioNTech/Genmab is testing the combination of GEN1046 with anti-PD1 Abs as a different, complementary mechanism of action (see above). However, these combinatorial approaches in the clinic will not unequivocally address the contribution of ICB component to PD-L1 x 4-1BB targeting approaches. Namely, a potentially observed synergistic activity might as well be explained by anti-PD1 Ab freeing up PD-L1 to binding by GEN1046 or by its additional blocking of PD-L2.
Most elegant way to get a definitive answer about the extent of ICB activity by PD-L1 x 4-1BB bsAb would be for BioNTech/Genmab to test GEN1046 in comparison to claudin 6 (CLDN6) x 4-1BB bsAb using similar models they developed (hCLDN6-expressing MDA-MB-231 cells and hCLDN6-expressing MC38).
Adjusting Potency in Design of Dual MOA bsAbs
If dual MOA is the goal of the immune checkpoint/agonist-targeting bsAbs, one of the ways to achieve it is by adjusting the potency of each of the components. This has been performed in the design of Tripokin (in development by Philogen), a fully-human immunostimulatory product consisting of TNF and IL2 fused into one molecular entity. It was reported that the TNF component of the agent was engineered with a mutation in order to reduce its potency to levels matching the IL-2 component so as to facilitate dose selection for the combined entity.
Adjusting potency will lead to the follow up generation of this class of bsAbs.